To build digital therapeutics platform solutions that can scale beyond pilots, companies must meet rapidly rising expectations for clinical and economic validation.
According to Mordor Intelligence, the Digital Therapeutics Device Market will grow from USD 9.96B in 2025 to USD 12.46B in 2026, reaching USD 38.19B by 2031, driven by software-based therapies integrated into real-world clinical workflows.
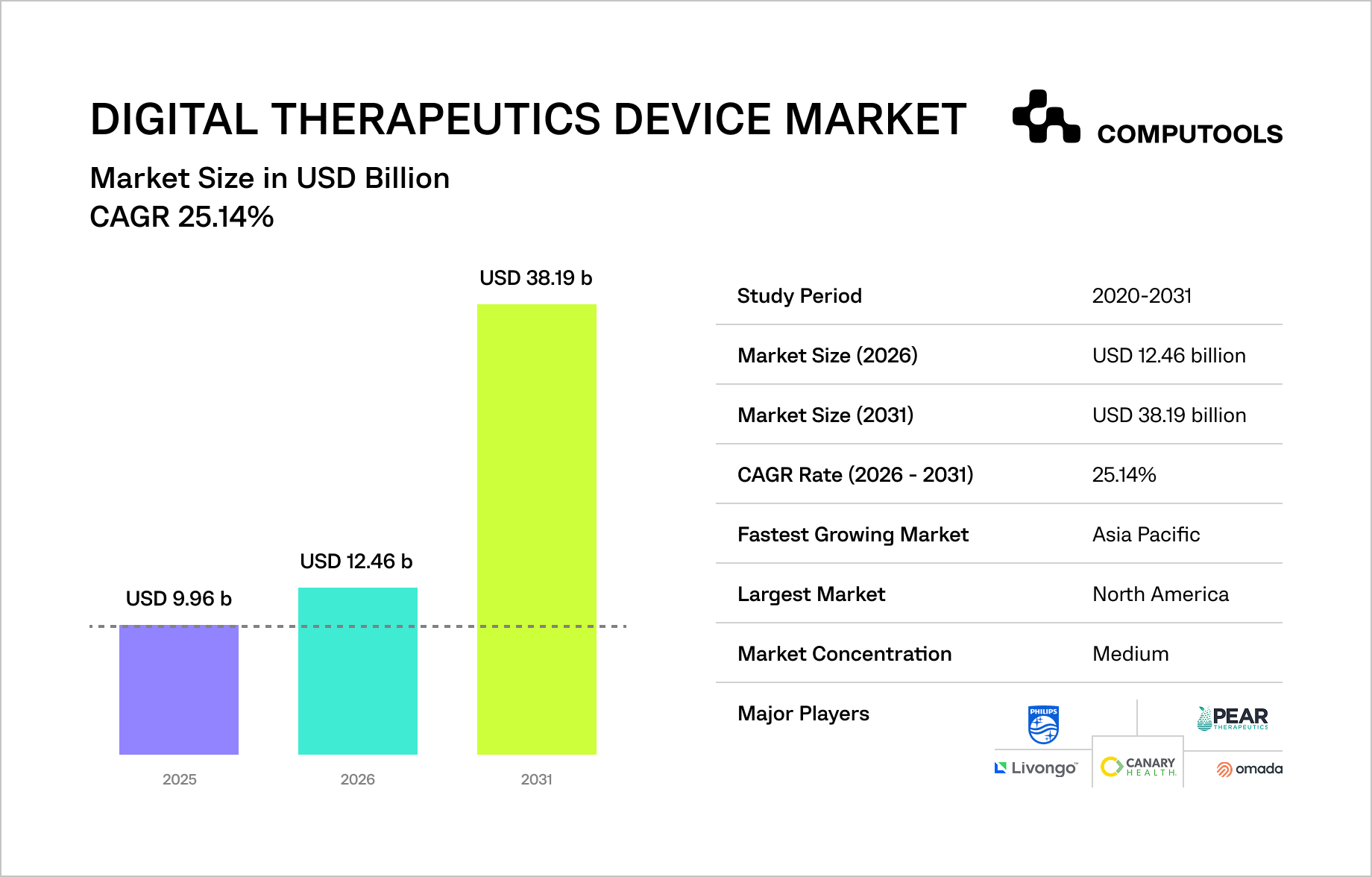
Clearer alignment between FDA regulatory requirements and CMS reimbursement criteria is reducing commercial uncertainty for digital therapeutics in the US, while Germany’s Digital Healthcare Act continues to shape European adoption. Consequently, success is now measured by the capacity to meet clinical evidence standards at scale, rather than just regulatory access.
According to Mordor Intelligence, software-only platforms accounted for 70.68% of the market in 2025; treatment applications accounted for 72.88%. Payers and insurers are the fastest-growing buyers, increasing pressure on platforms to demonstrate clinical value, reimbursement readiness, and long-term scalability from day one.
How we built a regulated healthcare platform without breaking clinical workflows
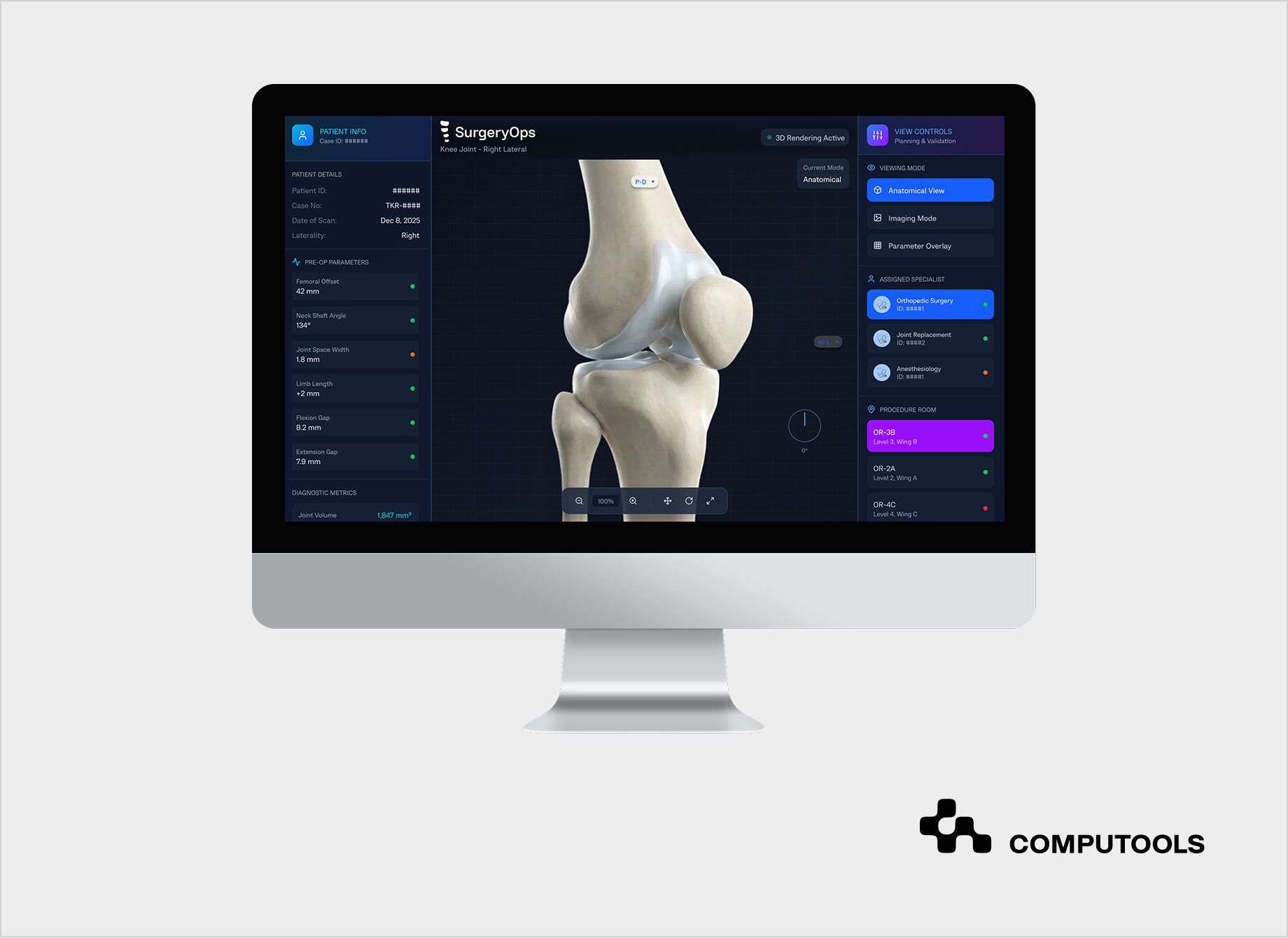
Our approach to building evidence-ready digital therapeutics platforms is grounded in real-world experience with regulated healthcare systems. We applied the same architectural principles while developing SurgeryOps, a secure operational platform for a multi-specialty hospital in the United States, where strict compliance, traceability, and workflow continuity were non-negotiable.
The platform was designed to support—not replace—clinical decision-making. We centralized scheduling and coordination workflows while preserving existing clinical processes, and embedded role-based access control, encryption, and full system-level auditability.
By separating operational logic from medical judgment, we ensured that automation improved consistency and visibility without influencing clinical outcomes, a critical requirement for products operating under clinical evidence standards and built through mature web platform development.
This experience directly informs how we build digital therapeutics platform solutions today. Evidence generation, AI governance, and compliance cannot be retrofitted after launch; they must be designed into the platform architecture from the start to ensure regulatory readiness and long-term adoption.
Based on this experience, we now move to a practical breakdown. Drawing on 12+ years of work with regulated healthcare systems, operating under ISO 9001 and ISO 27001, and delivering platforms compliant with GDPR and HIPAA, the following sections outline a step-by-step approach to building a digital therapeutics platform.
How to build digital therapeutics platform that meets clinical evidence standards
Step 1. Define the therapeutic scope and evidence intent before implementation
In digital therapeutics, implementation choices depend directly on what the therapy is expected to achieve clinically. If the therapeutic scope is vague, it becomes impossible to define valid outcomes, select appropriate endpoints, or design studies that withstand external review.
The first task is to clearly state the medical purpose of the solution, whether it supports treatment, disease management, or prevention, and to define the patient population and context of use within care delivery.
Clinical claims must be specific and testable, because they determine both the data that needs to be collected and the level of evidence required.
Evidence frameworks explicitly note that validation criteria vary by patient population, measurement method, and clinical context, which is why early generalization creates downstream risk.
Once the intended claims are clear, they should be translated into a structured set of outcomes. Primary outcomes establish whether the intervention achieves its intended effect, while secondary and exploratory outcomes provide supporting context. This hierarchy is critical when multiple endpoints are involved, as it prevents misinterpretation of results and aligns the product with regulatory and payer expectations.
This approach mirrors how we operate in practice. From the beginning, we designed the SurgeryOps platform to support operational planning only, without influencing medical decision-making. Outcomes focused on coordination efficiency and reducing errors, while access control, auditability, and data protection were considered fixed constraints.
Applying the same discipline to digital therapeutics—defining clear scope, making explicit claims, and setting predefined outcomes—establishes a stable foundation for evidence generation before any coding begins.
Step 2. Plan the evidence and regulatory path before starting development
Once the therapeutic scope and intended claims are defined, the next step is aligning them with evidence and regulatory expectations.
This is where many teams make a costly mistake: they start digital therapeutics software development assuming that evidence can be “added later,” when in reality it dictates how the system must be built from the start.
At this stage, the focus is on outlining a feasible evidence plan rather than finalizing a submission strategy. That includes understanding how the product is likely to be classified, what level of clinical understanding is required for its claims, and which outcomes will be scrutinized by regulators, payers, or clinical partners. Evidence planning here directly influences architectural decisions, what must be traceable, what must be locked, and what can evolve without invalidating prior results.
In practice, this means deciding early which parts of the system are evidence-critical. Data collection logic, endpoint calculations, user interaction flows tied to outcomes, and versioning rules must be stable and auditable. If these elements change arbitrarily during development, previously collected data may lose validity, forcing teams to restart studies or limit the use of results.
We applied the same logic in the SurgeryOps platform, even though it was not a therapeutic product. Planning workflows, access rules, and audit trails were defined before implementation to ensure operational outcomes remained comparable over time. That discipline, treating measurability and traceability as first-class requirements, is directly transferable to digital therapeutics, where evidence continuity is non-negotiable.
By the end of this step, teams should have a clear view of the evidence required, when it will be generated, and which parts of the platform are constrained by those requirements. Only after that does it make sense to move into architectural design.
Step 3. Define platform architecture constraints based on evidence requirements
After the evidence path is defined, architectural decisions become constrained by what must remain consistent over time. At this stage, DTx platform architecture cannot be structured around features or short-term product iterations. It must support stable measurement, outcome comparability, and traceability across versions.
The primary task is separating evidence-critical logic from changeable product layers. Outcome calculations, intervention rules, and data-collection timing must be decoupled from UI design, onboarding flows, and engagement mechanics.
This allows teams to evolve the product experience without affecting how results are generated or interpreted. When this separation is missing, even small changes can invalidate previously collected evidence.
Versioning and traceability are equally non-negotiable. Every outcome must be tied to a specific intervention version and configuration. This requires explicit version control for evidence-relevant logic, controlled rollout of changes, and auditable records of when and how updates occurred. Without this structure, teams often produce results that exist but cannot be reliably explained or defended.
We followed the same discipline in SurgeryOps. Scheduling rules, access logic, and coordination workflows were fixed early, while visual layers and dashboards evolved based on operational feedback.
As a result, performance metrics stayed comparable over time. The same constraint-first approach is required in digital therapeutics to preserve evidence integrity as the platform evolves.
Step 4. Implement security and compliance as system requirements
Security and compliance cannot be treated as external checks applied after development. In regulated healthcare, they define how data is stored, accessed, changed, and audited, which directly affects whether clinical evidence remains valid over time. This is where secure healthcare platform development becomes a prerequisite for evidence, not a parallel concern.
At this stage, teams need to formalize access boundaries and data handling rules. Role-based access must reflect real clinical and operational roles, not generic user types.
Sensitive actions: data changes, configuration updates, and intervention adjustments must be logged to support traceability and retrospective reviews. Encryption, consent handling, and data minimization should be enforced at the system level to protect evidence-related data without relying on process discipline alone.
From an evidence perspective, stability matters as much as protection. If access rules, consent logic, or data retention policies change without control, previously collected data may no longer meet regulatory or study requirements. This is why security controls, audit logging, and configuration management must be versioned and treated as part of the evidence baseline.
We followed the same approach in SurgeryOps. Access to patient-related scheduling data was strictly role-based, all sensitive actions were auditable, and operational logic changes were controlled to avoid disrupting reporting consistency. That structure ensured that improvements to the system did not compromise data integrity. The same principle applies to digital therapeutics: compliance mechanisms must preserve evidence continuity as the platform evolves.
Security and data governance remain foundational for any evidence-driven healthcare platform, particularly when AI and sensitive patient data are involved. Read more in our blog: How to Design HIPAA-Compliant AI Architecture for Healthcare Applications.
Step 5. Translate clinical requirements into measurable evidence
At this stage, platform decisions must be aligned with clinical evidence standards for digital therapeutics—not in abstract terms, but in how data is generated, structured, and interpreted. The key task is to ensure that the system’s measurements are clinically meaningful, reproducible, and appropriate for the intended use.
This starts with endpoint discipline. Outcomes must reflect how a patient feels, functions, or responds to therapy, not just product usage or engagement metrics. Primary endpoints need to be clinically relevant and sensitive to change, while secondary and exploratory endpoints must be clearly contextualized to avoid overstating results. The platform must enforce this structure technically, ensuring endpoints are collected consistently and in accordance with predefined protocols.
Equally important is controlling variability. Differences in data capture timing, user interaction patterns, or configuration changes can introduce noise that weakens evidence. To prevent this, evidence-related data flows should be standardized and insulated from routine product iteration. When real-world data is collected, it must be traceable to a specific intervention version and context so that results remain interpretable.
In SurgeryOps, this principle also applies to operational metrics. The team consistently tracked planning efficiency and conflict reduction over time, despite interface changes. By standardizing how outcomes were calculated and reported, they prevented data drift. In digital therapeutics, failing to do so has more serious consequences: evidence that cannot be confidently interpreted becomes unusable.
Step 6. Validate the intervention through controlled and real-world studies
After outcomes are defined and data flows are stabilized, the platform must support digital therapeutics clinical validation without ad-hoc adjustments. Validation is a sequence that demonstrates the intervention works as intended under defined conditions and remains interpretable as it scales.
The practical task here is to align the study design with how the platform operates. For higher-risk claims, controlled designs are often required; for others, comparative or pragmatic approaches may be acceptable.
Regardless of design, the system must enforce protocol fidelity: fixed endpoint definitions, consistent timing, and controlled configuration changes during study periods. This prevents drift between what was tested and what is later deployed.
Blinding, control arms, and version control introduce additional constraints. If a sham or comparator is used, the platform must support indistinguishable experiences where required and preserve assignment integrity. Any update that could influence outcomes must be gated or deferred until after data lock. These are engineering problems as much as clinical ones.
We applied similar controls in SurgeryOps when operational metrics needed to remain comparable across reporting periods. Changes to planning logic were isolated and made deliberately to avoid contaminating performance data. In digital therapeutics, the same discipline ensures that validation results remain defensible to regulators, clinicians, and payers.
For teams exploring AI-powered features in digital therapeutics or decision-support workflows, understanding regulatory limits and clinical responsibilities is crucial. Learn more in our blog: How to Build an AI Clinical Decision Support System Without Violating Regulations.
Step 7. Operationalize clinical trials without breaking the product
When moving into formal studies, the platform must support clinical trials for digital therapeutics platforms as a controlled operating mode, not as a parallel, custom-built version of the product. The main risk at this stage is fragmenting the system—one version “for trials” and another “for real users”— which almost always leads to inconsistencies and unusable evidence.
The platform should support trial-specific configurations while preserving the same core logic used in production. This includes controlled enrollment, protocol-specific feature flags, fixed endpoint definitions, and clear rules for what can and cannot change during an active study. Data capture must follow the protocol exactly while reflecting how the intervention is used in practice, not under idealized conditions.
Equally important is managing the transition between study phases and live operation. Once a trial concludes, teams need a clean way to lock data, document the tested configuration, and continue product evolution without contaminating validated results. If this boundary is not technically enforced, subsequent updates may undermine earlier findings.
We used the same mindset in our case when reporting operational outcomes across defined time windows. Planning logic was frozen for measurement periods, and changes were introduced only between reporting cycles. For digital therapeutics, this approach enables rigorous trial execution while keeping the product on track for real-world deployment.
Step 8. Scale the platform while preserving evidence integrity
After validation and initial studies, the challenge shifts from proving that the intervention works to ensuring it continues to work as the platform scales. At this stage, the goal is to operate a digital health platform, where real-world use strengthens the original clinical claims.
The main risk here is uncontrolled change. As user volumes grow, teams inevitably introduce optimizations: onboarding tweaks, engagement adjustments, and performance improvements.
Without clear boundaries, these changes can alter how outcomes are produced, making post-launch data incomparable to trial results. To prevent this, evidence-relevant logic must remain versioned and protected, while real-world evidence collection follows predefined rules tied to specific configurations.
Real-world evidence should extend, not replace, clinical findings. Usage patterns, adherence signals, durability of response, and safety indicators must be interpreted in the context of the validated intervention, not as standalone success metrics. This requires disciplined data governance and the ability to link outcomes back to the exact intervention version in use at the time.
In SurgeryOps, we applied the same principle when the platform expanded across departments and operational load increased. Metrics were kept comparable by anchoring reporting to stable logic, even as scale and complexity grew. For digital therapeutics, this approach allows teams to grow adoption while maintaining credibility with regulators, clinicians, and payers.
Step 9. Prepare the platform for regulatory review and ongoing compliance
At this stage, the platform must be ready to withstand external review. Preparing FDA compliant digital therapeutics software means ensuring that evidence, system behavior, and documentation remain aligned throughout the product lifecycle.
Validated clinical validation must match deployment, with deviations explainable and controlled, requiring tight linkage between versioning, documentation, and change management. Regulators expect a clear link from clinical claims to system behavior, supported by traceable data, logs, and rationale.
Post-market monitoring of safety, performance, and effects is also vital for compliance. Updates impacting logic or outcomes need regulatory assessment to avoid invalidating evidence or re-review.
In SurgeryOps, a similar discipline was applied to operational compliance. Changes to scheduling logic or access rules were documented, reviewed, and released in a controlled manner to preserve auditability and reporting integrity. For digital therapeutics, the same rigor ensures regulatory readiness is sustained over time rather than treated as a one-time milestone.
Step 10. Align delivery, evidence, and operations into a single system
At this point, success depends on whether digital therapeutics platform development is treated as a continuous system rather than a sequence of disconnected phases. Clinical claims, evidence generation, regulatory readiness, and day-to-day operations must reinforce one another rather than compete for priority.
The core task is alignment: product delivery must respect evidence constraints, and workflows should mirror platform operation in clinical and real-world settings. This includes synchronized release management, clear ownership of evidence-critical components, and shared accountability among engineering, clinical, and compliance teams. When these functions diverge, platforms either stagnate due to regulatory fear or evolve too quickly, invalidating evidence.
From an execution standpoint, this is where mature healthcare software development for digital therapeutics makes a difference. Teams must be able to ship updates, respond to clinical feedback, and scale adoption while preserving traceability, auditability, and comparability of outcomes. Evidence becomes a living asset—maintained, extended, and defended as the platform grows.
This is the same systems-level thinking we applied in SurgeryOps: operational workflows, metrics, and compliance controls were designed to evolve together rather than in isolation. For digital therapeutics, the stakes are higher, but the principle is the same. Platforms that integrate delivery, evidence, and operations remain credible—to clinicians, payers, and regulators—long after launch.
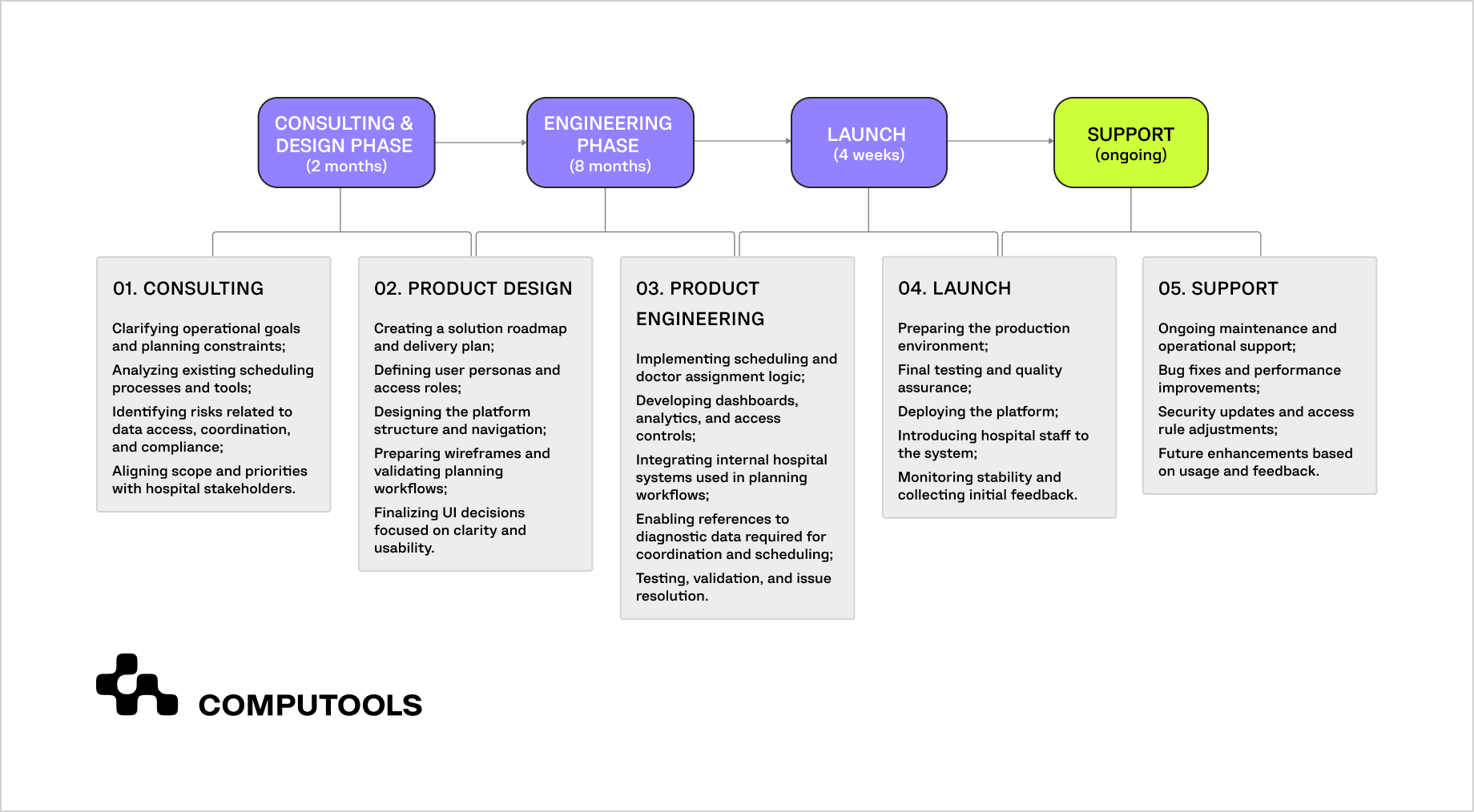
The steps above outline how a digital therapeutics platform should be structured to support clinical evidence generation from the start. Defining the therapeutic scope, stabilizing evidence-critical logic, enforcing security and traceability, and validating interventions through controlled and real-world use are interdependent parts of a single system.
In practice, these requirements are difficult to meet without delivery processes already adapted to regulated healthcare environments. This is where healthtech software development services focused on long-term compliance, controlled change, and evidence continuity play a practical role—supporting platform evolution without compromising clinical validity.
With governance and evidence architecture defined, the next question is what functional components such a platform must include.

Define the roadmap for regulatory-grade security, patient data governance, and outcomes tracking—engage experienced healthtech architects for a detailed project assessment.
What are the core features of a digital therapeutics platform?
A digital therapeutics platform must support both therapy delivery and evidence generation. The features below are required to operate a digital health platform with clinical evidence, rather than a generic health application.
• Patient-facing functionality centers on structured therapy execution. A patient dashboard provides access to therapy modules, daily tasks, reminders, and progress tracking tied to clinically defined outcomes. Wearable integrations or structured manual inputs enable continuous data capture, while educational content supports correct use of the therapy without replacing clinical guidance.
• Clinician-facing tools focus on visibility and oversight. Clinician dashboards aggregate patient data into interpretable reports, highlight deviations or risk signals, and support communication without increasing administrative burden. Analytics must be aligned with predefined endpoints, ensuring clinicians see validated measures rather than raw engagement metrics.
• At the core of the platform are evidence-based digital therapeutics solutions—clinically grounded intervention modules derived from medical guidelines and trial protocols. These modules must be versioned, traceable, and insulated from UI or engagement-layer changes to preserve evidence integrity over time.
• Personalization mechanisms, including rule-based logic or AI-driven recommendations, adapt therapy within approved clinical boundaries. Any adaptation must be explainable and auditable, especially when it affects outcome-related behavior or exposure intensity.
• Secure communication tools (chat, messaging, and video) enable patient–provider interaction when required, while maintaining clear boundaries between therapeutic support and medical decision-making.
• Finally, security is a condition. Data encryption, role-based access control, audit logging, and controlled configuration changes are essential to maintain digital therapeutics regulatory compliance and to ensure that clinical evidence remains valid as the platform evolves.
What are the common feature mistakes that undermine clinical evidence
Many digital therapeutics initiatives fail because feature decisions are made without considering how evidence is generated, used, and preserved. Below are the most common mistakes we see during digital therapeutics product development, especially when teams move too quickly from concept to implementation.
1. Treating engagement features as primary outcomes.
Dashboards filled with streaks, badges, and session counts often replace clinically meaningful measures. Engagement can support therapy, but when it becomes the main indicator of success, platforms struggle to demonstrate real clinical impact or align results with predefined endpoints.
2. Blending intervention logic with UI behavior.
When therapy rules, dosing logic, or progression criteria are tightly coupled to interface flows, even small UX changes can alter how the intervention works. This makes it difficult to compare results across versions or explain outcome variability during reviews.
3. Over-personalization without clinical boundaries.
Adaptive recommendations can improve short-term adherence, but when introduced through AI development in healthcare without clear constraints, explainability, and auditability, they create uncontrolled variability. This makes it difficult to interpret outcomes or ensure consistent exposure across patient cohorts.
4. Building clinician dashboards from raw data instead of evidence models.
Providing clinicians with unfiltered metrics and alerts that are not tied to validated endpoints increases cognitive load and the risk of misinterpretation. Evidence-driven platforms require structured views that reflect clinical intent, not just data availability.
5. Adding communication tools without role clarity.
Chat and messaging features often blur the line between therapeutic support and clinical decision-making. Without explicit boundaries, these tools introduce operational and regulatory risk and complicate evidence interpretation.
6. Postponing security and governance decisions.
Access control, audit logging, and configuration management are sometimes treated as infrastructure details to be finalized later. In practice, late changes to these mechanisms can compromise data integrity and limit the usability of previously collected evidence.
Avoiding these mistakes requires better alignment between product decisions and evidence strategy. Platforms that focus on clarity, separation of concerns, and controlled change are more likely to sustain credible clinical outcomes as they evolve.
Why organizations choose Computools
Organizations building digital therapeutics and healthcare data platforms choose Computools because we design systems that remain stable under clinical, regulatory, and operational pressure. With 250+ engineers and 400+ delivered projects, we bring engineering depth beyond feature delivery.
We operate under ISO 9001 and ISO 27001 and build solutions compliant with GDPR and HIPAA, embedding security, auditability, and controlled change at the system level. Our work is trusted by organizations such as Visa, Epson, and IBM and supported by partnerships with Microsoft and AWS.
In healthcare projects, we focus on governed data and workflow integrity, secure ingestion layers, versioned data models, and audit-ready architectures that scale without disrupting clinical systems. This approach underpins our hospital software development services, where platforms must integrate with EHRs and remain compliant as usage grows.
Across regulated projects, we have delivered up to 45% faster patient data processing and 40% reductions in administrative overhead through workflow automation.
In SurgeryOps and other healthcare platforms, from equipment tracking to medical imaging and medication adherence, the same principles ensured data integrity, clinical autonomy, and reliable operation at scale.
What differentiates Computools is consistency in execution. We build healthcare platforms as long-term capabilities that support evidence generation, regulatory review, and operational efficiency.
If you are planning or reworking a digital therapeutics platform and want to discuss technical and architectural considerations, contact our team at info@computools.com.
Choosing a technology partner for regulated healthcare requires more than feature expertise, which is why we reviewed the landscape of leading vendors in the Top 25 Healthtech Software Development Companies in 2026.

Computools
Software Solutions
Computools is a digital consulting and software development company that delivers innovative solutions to help businesses unlock tomorrow.








“Computools was selected through an RFP process. They were shortlisted and selected from between 5 other suppliers. Computools has worked thoroughly and timely to solve all security issues and launch as agreed. Their expertise is impressive.”